Many cancer biomarkers in the bood get damaged during long term storage when kept at cryogenic temperatures due to stresses associated with ice formation and low temperatures. The aim of this research is to develop isothermal vitrification methods to ensure that the archival serum samples are stably stored at room temperature for a long time.

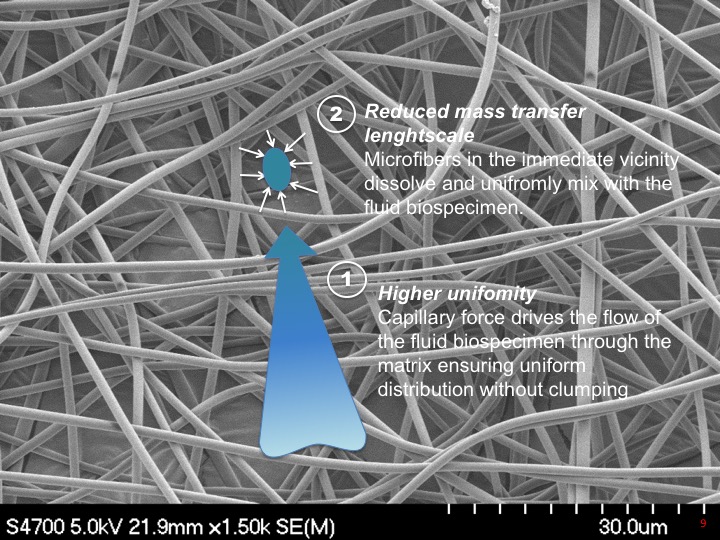
We have developed a lyoprotectant matrix that is used for stabilizing archival serum samples. Serum is pipetted into the wells filled with the lyoprotectant matrix, and dried overnight in a vacuum chamber, which vitrifes the sample making it stable during room temperature storage. The matrix is an electrospun non-woven fibrous material electrospun from a patent pending lyoprotectant solution. The capillary forces help adsorb the serum into the matrix, and dissolve to uniformly load the serum with the lyoprotectants, avoiding clumping.
The results of the initial validation studies we have conducted have recently been published [Scientific Reports, 6, 24186, 2016];
We are currently in the process of collecting longer term storage results after making some modifications to the existing matrix. Stay tuned!